Information about the Fire Triangle/Tetrahedron and Combustion
To understand how fire extinguishers work, you need to know about combustion. It is impossible in this short introduction to completely describe all the complex chemical and physical reactions that take place during a fire. However, this page will offer an introduction into the fundamental theories of fire and explosion.
The Fire Tetrahedron (A pyramid)
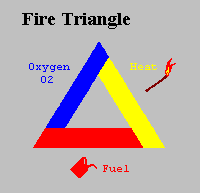 For many years the concept of fire was symbolised by the Triangle of Combustion and represented, fuel, heat, and oxygen. Further fire research determined that a fourth element, a chemical chain reaction, was a necessary component of fire. The fire triangle was changed to a fire tetrahedron to reflect this fourth element. A tetrahedron can be described as a pyramid which is a solid having four plane faces. Essentially all four elements must be present for fire to occur, fuel, heat, oxygen, and a chemical chain reaction. Removal of any one of these essential elements will result in the fire being extinguished.
For many years the concept of fire was symbolised by the Triangle of Combustion and represented, fuel, heat, and oxygen. Further fire research determined that a fourth element, a chemical chain reaction, was a necessary component of fire. The fire triangle was changed to a fire tetrahedron to reflect this fourth element. A tetrahedron can be described as a pyramid which is a solid having four plane faces. Essentially all four elements must be present for fire to occur, fuel, heat, oxygen, and a chemical chain reaction. Removal of any one of these essential elements will result in the fire being extinguished.
The four elements are oxygen to sustain combustion, sufficient heat to raise the material to its ignition temperature, fuel or combustible material and subsequently an exothermic chemical chain reaction in the material. Each of the four sides of the fire tetrahedron symbolise the Fuel, Heat, Oxygen and Chemical Chain Reaction. Theoretically, fire extinguishers put out fire by taking away one or more elements of the fire tetrahedron.
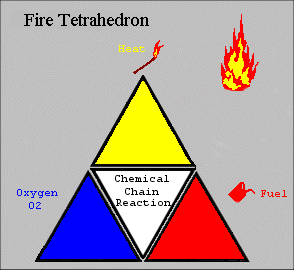 This model, although simplistic, is a good analogy as to the theory of how to extinguish a fire. For example, a foam extinguisher would create a barrier around the combustible materials and cut off the supply of oxygen as well as reducing heat. By applying water you can lower the temperature below the ignition point, or for a flammable liquid fire it would remove or divert the fuel. Finally, interfering with the chemical chain reaction is possible by “mopping up” the free radicals in the chemical reaction using BCF and other halon extinguishers, which also create an inert gas barrier. However, this type of extinguisher is being phased out and in the future other extinguishing agents may be found using this principle. The 2D figure opposite represents a 3D model of a tetrahedron.
This model, although simplistic, is a good analogy as to the theory of how to extinguish a fire. For example, a foam extinguisher would create a barrier around the combustible materials and cut off the supply of oxygen as well as reducing heat. By applying water you can lower the temperature below the ignition point, or for a flammable liquid fire it would remove or divert the fuel. Finally, interfering with the chemical chain reaction is possible by “mopping up” the free radicals in the chemical reaction using BCF and other halon extinguishers, which also create an inert gas barrier. However, this type of extinguisher is being phased out and in the future other extinguishing agents may be found using this principle. The 2D figure opposite represents a 3D model of a tetrahedron.
A Definition of Fire
One generally accepted definition of combustion or fire, is a process involving rapid oxidation at elevated temperatures accompanied by the evolution of heated gaseous products, and the emission of visible and invisible radiation. Oxidation occurs all around us in the form of rust on metal surfaces, and in our bodies by metabolising the food we eat. However, the key word that sets combustion apart from other forms of oxidation is the word “rapid”.
The combustion process is usually associated with the oxidation of a fuel in the presence of oxygen with the emission of heat, light, and other exhaust products. Oxidation, in the strict chemical sense, means the loss of electrons. For an oxidation reaction to occur, a reducing agent (the fuel) and an oxidising agent (usually oxygen) must be present. As heat is added, the ignition source, fuel molecules, and oxygen molecules gain energy and become active. This molecular energy is transferred to other fuel and oxygen molecules which creates a chain reaction where the fuel loses electrons and the oxygen gains electrons. This exothermic electron transfer emits heat and/or light. If the fire is in a fire grate or furnace we refer to this process as a controlled fire, and if it is a building on fire we refer to this process as an uncontrolled fire.
The Combustion Modes
The combustion process occurs in two modes:
- Flaming
- Non-flaming, smouldering or glowing embers.
For the flaming mode it is necessary for solid and liquid fuels to be vaporised. The solid fuel vapours are thermally driven off, or distilled and the liquid fuel vapours evaporated. It is this volatile vapour from the solid or liquid fuels that we see actually burning in the flaming mode. This gas or vapour production emitted from the fuel is referred to as pyrolysis. Once a flame has been established, heat transfer from the flame to the fuel surface continues to drive off more volatile gases and perpetuates the combustion process. Continued burning in the flaming mode requires a high burning rate, and the heat loss associated with transfer of heat from the flame area by conduction, convection, and radiation must be less than the energy output of the fire. If the heat loss is greater than the energy output of the fire, the fire will extinguish.
Both modes, flaming and non-flaming, can occur separately or in combination. Flammable liquids and gases only burn in the flaming mode. Wood, straw, and coal are examples where both modes may exist simultaneously.
Flaming combustion can occur in the following forms:
- Premixed flames where the fuel and oxygen are mixed prior to ignition. For example the flame on a Bunsen burner, gas stove, or propane torch.
- Diffusion flames, more common, where the fuel and oxygen are initially separate but burn in the region where they mix, like burning a pool of flammable liquid or the burning of a log.
Stages of a Fire
There are three generally recognised stages to a fire. The incipient stage, smouldering stage, and flame stage.
The incipient stage is a region where preheating, distillation and slow pyrolysis are in progress. Gas and sub-micron particles are generated and transported away from the source by diffusion, air movement, and weak convection movement, produced by the buoyancy of the products of pyrolysis.
The smouldering stage is a region of fully developed pyrolysis that begins with ignition and includes the initial stage of combustion. Invisible aerosol and visible smoke particles are generated and transported away from the source by moderate convection patterns and background air movement.
The flaming stage is a region of rapid reaction that covers the period of initial occurrence of flame to a fully developed fire. Heat transfer from the fire occurs predominantly from radiation and convection from the flame.
Classes of fire
Combustible and flammable fuels involved in fires have been broken down into five categories:
- Class A fires – are fires involving organic solids like paper, wood, etc.
- Class B fires – are fires involving flammable liquids
- Class C fires – are fires involving flammable gasses
- Class D fires – are fires involving metals
- Class F fires – are fires involving cooking oils.
Summary
A fire begins by an external ignition source in the form of a flame, spark, or hot ember. This external ignition source heats the fuel in the presence of oxygen. As the fuel and oxygen are heated, molecular activity increases. If sufficiently heated, a self-sustaining chemical chain reaction or molecular activity occurs between the fuel and oxygen. This will continue the heating process and the resulting chain reaction will escalate without the need for an external ignition source. Once ignition has occurred, it will continue until:
- all the available fuel or oxidant has been consumed, or
- the fuel and/or oxygen is removed, or
- the temperature has been reduced sufficiently, or
- the number of excited molecules has been reduced significantly, breaking the chain reaction.
Explosions
Generally, an explosion is defined as a very rapid release of high-pressure gas into the environment. The energy from this very rapid release of the high-pressure gas is dissipated in the form of a shock wave.
Explosions can be classified as physical (a balloon bursting), as physical and/or chemical (a boiler explosion), or a chemical reaction of a gas/particle mixture. Our discussion will focus on chemical reaction explosions.
The process of a chemical reaction explosion is similar to the combustion process whereby a fuel and oxidising agent have mixed prior to ignition, such as petroleum vapour or fine particles of grain dust mixed with air. However, in an explosion the oxidation process proceeds at a greatly accelerated rate. The oxidation process is usually, but not always, confined within an enclosure, such as a tank or grain silo, so that a rapid high-pressure rise occurs with an associated flame front. Generally, it is this high-pressure shock wave that causes the damaging effects from an explosion.
Resultant shock waves that propagate from the point of ignition at a velocity less than the speed of sound are termed deflagration. Shock wave velocities in excess of the speed of sound are termed detonations.
A rise in pressure creating a shock wave of 6894.76 Pascals is sufficient to knock a person down. If the rise in pressure creates a shock wave of 13789.52 Pascals to 20684.28 Pascals, this is sufficient to shatter an 8 to 12-inch thick concrete wall. A Pascal (Pa) is equivalent to 1 N/m2 (one newton per square metre).
Categories:Miscellaneous Fire Safety Issues
April 1, 2011[Last updated: February 10, 2022]
Comments are closed here.